-40%
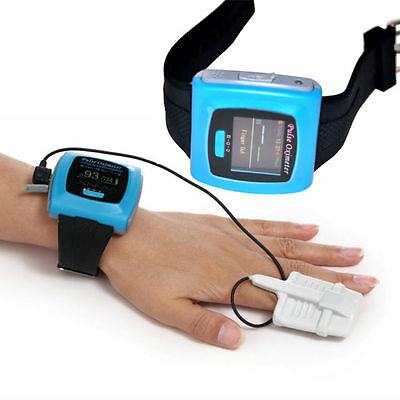
Wrist Pulse Oximeter,Spo2 Monitor Daily And Overnight Sleep Wearable Monitor FDA
$ 48.84
- Description
- Size Guide
Description
Wrist Pulse Oximeter,Spo2 Monitor Daily And Overnight Sleep Wearable Monitor FDAInstructions
Principle of the CMS50F Pulse Oximeter is as follows: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning & Recording Technology,the Pulse Oximeter can be used in measuring the pulse oxygen saturation and pulse rate through finger.The product is suitable for being used in family, hospital, oxygen bar, community healthcare, physical care in sports (It can be used before or after doing sports, and it is not recommended to use the device during the process of having sport) and etc.
Major Features
1. Small in volume、light in weight and convenient in carrying
2. Operation of the product is simple, low power consumption
3. SpO
2
value display
4. Pulse rate value display, bar graph display
5. Pulse waveform display
6. The display mode can be changed
7. Screen brightness can be changed
8. A pulse rate sound indication
9. With measured data overruns limits and low-voltage alarm function, the upper/down alarm range can be adjustable
10. With clock function
11. Battery capacity indication
12. Low-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltage, and with alarm function
13. With SpO
2
value and pulse rate value of storage, the storage data can be uploaded to computers
14. Real-time data can be transmitted to computers(Only for the Lei Mo probe)
15. Connected with an external oximeter probe
Main performance
· Display Mode: 1.3" 65K Color OLED display
· Screen Resolution: 128*96
· SpO2 Measuring Range: 0%~100%(the resolution is 1%)
· Accuracy: 70%~100%: ±2%, Below 70% unspecified.
· PR Measuring Range: 30bpm~250bpm(the resolution is 1bpm)
· Accuracy: ±2bpm or ±2% (select larger)
· Measurement Performance in Weak Filling Condition:SpO
2
and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO
2
error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger).
· Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
· Power Consumption: less than 100mA
· Voltage: DC 3.6V~4.2V
· Power Supply: Voltage 3.7 rechargeable lithium battery × 1
· Battery working hour: The theoretical number is 20 hours
· Battery working life: Charge and discharge no less than 500 times.
· Safety Type:Interior Battery, BF Type
Accessories
· Sell in standard
A user manual
A USB line
a wristband (Only for the Lei Mo probe)
A disk (PC software)
An adult-oximeter probe
A power adapter
· Sell in addition
Other oximeter probe
Physical Identity
Dimension: 61(L) × 56(W) × 24 (H) mm
Weight: About 60g (with the lithium battery*1)
Certification
CE, FDA
Payment:
*We accept payment from PAYPAL, Bank transfer,
Visa,Credit Card and others.
*All payments are expected within 7 days after the last
winning auction is closed.
Shippment
*
Clearance: we will ship your item(s) to your PayPal confirmed address.Please leave me your clear
address,telephone,postal code.
*
All items will be dispatched within 2 bussiness day.
* The item will be declared as lower value for shipping only.We'd like to cooperate with you and declare the value of it
as
you want us to, so that it is more easier for you to clear the custom.
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for
REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state
and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an
authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with
the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with
the code 197923,
and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse
Oximeter that is FDA 510K Approved
Only English user guide,if you need any other language,please contact us!
Track Page Views With
Auctiva's FREE Counter